In every field, there are a few tests that separate claims from reality. In genomic evidence, that test is TP53.
TP53 appears everywhere. It’s the archetypal tumor suppressor, altered across a wide range of solid tumors and hematologic malignancies. It also sits at the center of hereditary cancer syndromes like Li-Fraumeni, where a single germline variant can reshape a family’s risk profile, surveillance plan, and long-term strategy.
Anyone can say they have “comprehensive coverage.” TP53 is where that claim is either proven or exposed.
At Genomenon, TP53 is extensively curated across both our germline and somatic solutions - The Cancer Knowledgebase (CKB) for somatic oncology and Mastermind Genomic Intelligence Platform for germline interpretation. Together, they turn TP53 into a concrete example of what extensive coverage looks like when it’s actually delivered, not just promised.
And through the end of the year, TP53 will be available to explore at no cost in both CKB and Mastermind, so your team can see that coverage in action, on your own terms.
Somatic TP53 in CKB: from variants to therapies and trials
In oncology, TP53 is no longer just about risk; it’s about strategy - tumor biology, therapeutic opportunity, and trial positioning.
Somatic TP53 is captured in three connected layers so teams can move directly from a mutation call to concrete options.
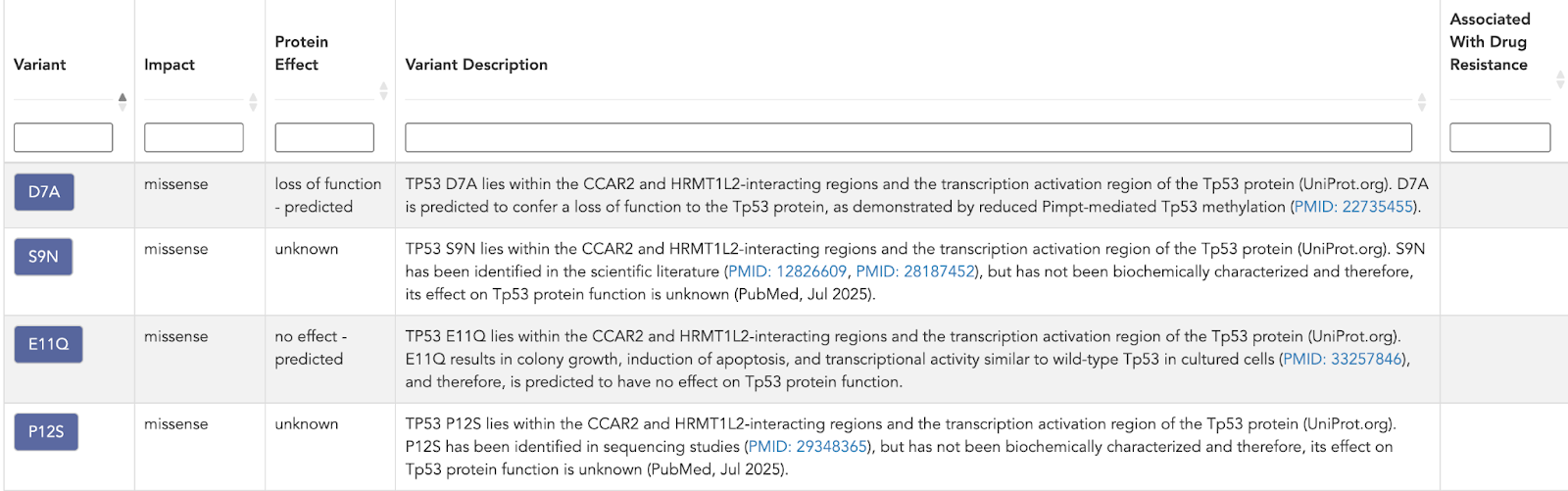
Variant layer
TP53 is represented by 795 somatic variants, each with structured annotations for:
- Variant - the exact genomic/protein change
- Impact
- Protein effect
- Variant description - concise, literature-grounded context
This replaces a generic “TP53 mutated” flag with precise, actionable alteration data. You know not just that TP53 is altered, but how it is altered and what that implies.
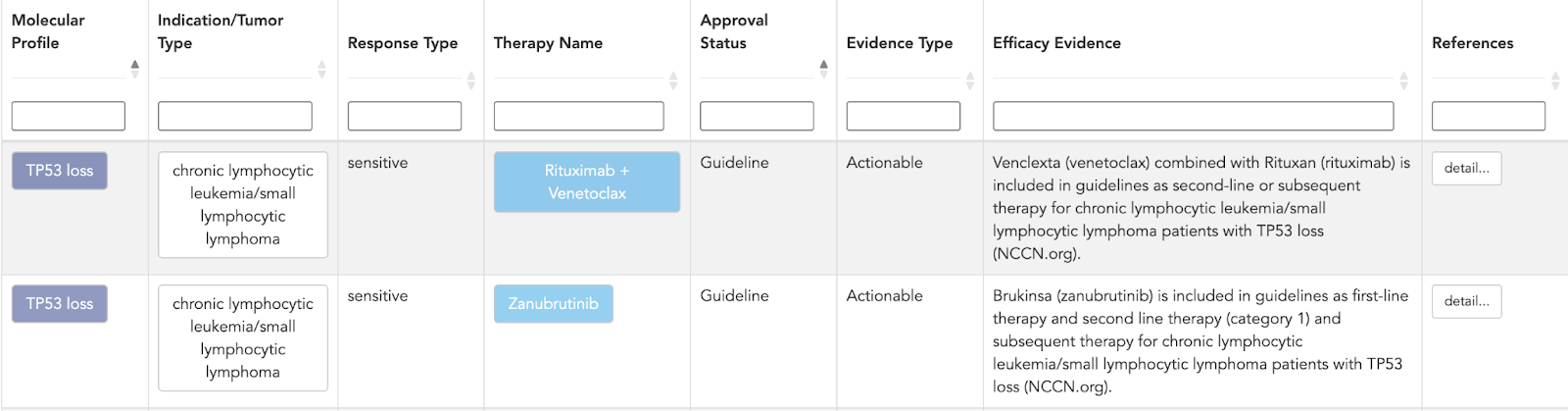
Molecular profile layer
CKB then groups TP53 into molecular profiles that reflect real clinical contexts, including:
- Indication / tumor type
- Response type - sensitivity, resistance, prognostic impact, lack of benefit
- Therapy name
- Approval status
- Evidence type - clinical trial, case report, retrospective study, etc.
- Efficacy evidence
This directly links TP53-driven profiles to observed therapeutic outcomes, so you’re not guessing which TP53 scenarios matter - you’re looking at where the evidence already shows an effect.
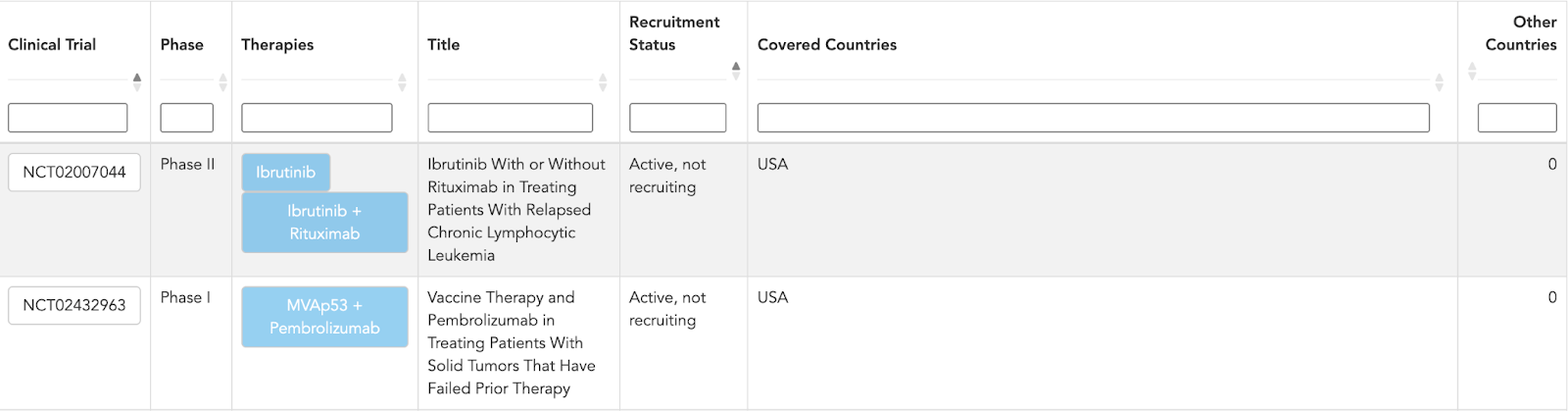
Clinical trial layer
Finally, TP53 is tied into the global clinical trial landscape, with entries for:
- Clinical trial ID and title
- Phase
- Therapies under evaluation
- Recruitment status
- Covered countries
This makes it immediately clear where TP53 is being investigated in study design - whether as an inclusion or exclusion criterion, a biomarker, or an exploratory endpoint - and where those studies are running.
Taken together, these layers turn somatic TP53 from a “frequently mutated gene” into a structured, evidence-backed framework for biomarker strategy, therapy selection, and trial planning.
Germline TP53 in Mastermind: from risk to reclassification
Powerful decisions start with the right foundation. In the germline setting, TP53 is the foundation for hereditary cancer workups.
In Mastermind, TP53 isn’t simply “present” in the database - it’s systematically assessed at both the gene-disease and variant level.
For TP53, Mastermind currently includes:
- 5 curated gene-disease relationships, evaluated using Genomenon’s Gene-Disease Relationship Assessment Standards:
- Li-Fraumeni syndrome - Definitive, Autosomal Dominant
- Colorectal cancer - Limited, Autosomal Dominant
- Familial ovarian cancer - Limited, Autosomal Dominant
- Basal cell carcinoma 7 - No Known Association, Autosomal Dominant
- Glioma susceptibility 1 - No Known Association, Autosomal Dominant
- Li-Fraumeni syndrome - Definitive, Autosomal Dominant
- Thousands of TP53 variants classified across variant types, each backed by the underlying literature
Behind each gene-disease relationship is a structured body of clinical and functional evidence - case series, pedigrees, functional assays - tied back to PubMed IDs and extracted into a consistent framework. Instead of hunting through scattered papers, you’re working from a curated evidence map.
This structure is particularly decisive for TP53 VUSs and borderline cases. When a family is trying to understand their risk, one additional case report, a new functional study, or a clarified gene-disease relationship can change the classification outcome. By combining curated gene-disease assessments with thousands classified variants, Mastermind helps move TP53 decisions away from “insufficient data” and toward confident, defensible calls - the kind of decisions that stand up to both clinical scrutiny and long-term consequences.
Why a unified germline + somatic TP53 view shifts the balance
Treating germline and somatic TP53 as separate worlds works only when the questions are narrow. In reality, clinical and pharma workflows are rarely that clean:
- Patients with a germline TP53 variant often go on to develop tumors where somatic events, co-mutations, and treatment history drive the next set of decisions.
- The same TP53 variant can surface in both germline and somatic contexts, and understanding both sides can refine risk management, surveillance, and therapeutic planning.
- Biopharma teams increasingly need to understand how hereditary risk, tumor evolution, and treatment response intersect around high-impact genes like TP53.
We often talk about “full coverage” of a gene. TP53 shows what that actually requires:
- Breadth - capturing the global literature across decades, not just a few high-level papers.
- Depth - going beyond mentions to extract variant-level, patient-level, and outcome-level detail.
- Continuity - linking germline and somatic use cases instead of treating them as disconnected workstreams.
A unified TP53 view across Mastermind and CKB gives your team the ability to navigate all three dimensions at once. Instead of reacting piecemeal to individual variants or isolated study results, you’re operating from a coherent evidence position - one that spans risk, diagnosis, treatment, and trial design.
If you’re working with TP53 - whether you’re interpreting hereditary cancer panels, designing oncology trials, or exploring new indications - we’d love to show you what TP53 without blind spots looks like in Mastermind and CKB, especially while TP53 is available to explore at no cost through the end of the year.