Transforming Drug Development with Literature-Driven Real-World Evidence
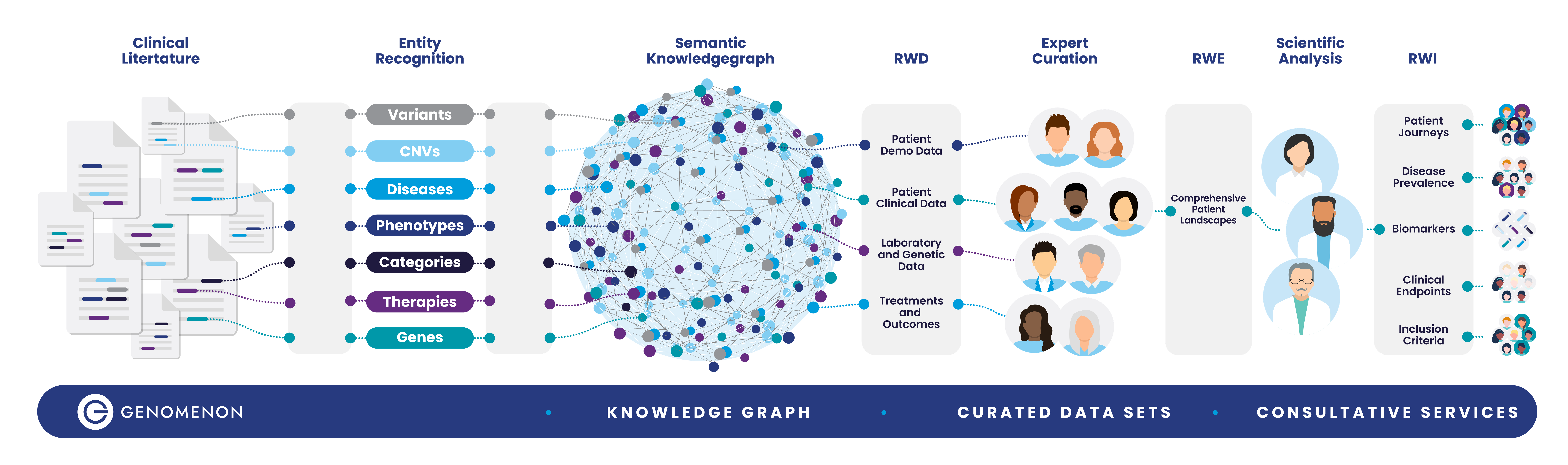
Genomenon’s Real-World Evidence for Precision Therapeutics leverages the company’s AI-powered knowledge graph to extract real-world data (RWD) from scientific literature. This data is then curated by a team of over 100 expert scientists into actionable RWE datasets or analyzed even further for real-world insights.
Unlike traditional methods that rely on electronic health records, Genomenon's RWE approach unlocks the vast repository of published research. By capturing trillions of dollars' worth of insights into rare disease and cancer patient presentations, clinical journeys, treatments, and outcomes, Genomenon’s RWE provides a unique upstream signal detection layer that complements existing RWE sources.
At every stage of development, Genomenon’s literature-driven RWE delivers a competitive advantage - whether making critical trial decisions, expanding disease insights, or identifying opportunities for label expansion. By accelerating timelines and strengthening evidence generation, our RWE equips pharma teams with actionable intelligence to de-risk investments, maximize pipeline potential, and bring therapies to patients faster.
RWE for Precision Therapeutics
The Genomenon Advantage


AI-Powered, Human-Validated Curation
Genomenon's AI platform rapidly identifies and extracts hundreds of genetic and clinical data points from more than 10 million scientific articles. All data is classified using industry-standard criteria and a team of experts reviews the data to ensure accuracy.

Comprehensive & Flexible Data
We know the importance of understanding the full range of diseases. Our datasets can include phenotypes, clinical outcomes, interventions, and even longitudinal data of providing unprecedented visibility into natural history, patient diversity, and real-world disease complexity for more precise inclusion/exclusion criteria and endpoint modeling.

Regulatory-Grade Transparency
All data is delivered with reference-cited and traceable to primary literature sources, supporting direct responses to regulatory and scientific partners. Genomenon provides not just raw data, but also a comprehensive executive summary, prevalence methodology, and regulatory review materials-empowering the sponsor to confidently answer questions from regulators, investigators, and collaborators with audit-ready evidence.

“Genomenon’s innovative approach to leveraging literature for real-world evidence significantly enhanced our understanding of ENPP1 Deficiency. The data they extracted and the evidence they gathered, not only deepened our knowledge of the disease landscape but also informed our strategic investment decisions, underscoring the power of real-world data in shaping market strategies for rare diseases.”
Catherine Nester, Senior Vice President, HCP and Patient Engagement at Inozyme Pharma
Powering Smarter Decisions Across the Drug Lifecycle
Clinical trials succeed when they’re built on evidence that matters. Genomenon empowers therapeutic developers with real-world insights to design smarter trials, reduce risk, and accelerate breakthrough therapies.

Unlocking new markets requires evidence that goes deeper than claims and EHR data. Genomenon empowers developers with precise, biologically grounded RWE to identify untapped patient populations, support FDA approval, and expand treatment impact.
Clear disease insights are the foundation of better decisions. Genomenon delivers comprehensive, evidence-backed RWE to define subtypes, uncover hidden populations, and refine guidelines with confidence.
Looking to advance awareness and improve diagnostic rates for your target rare disease?
Genomenon’s Genetic Disease Sponsorship reduces barriers to diagnosis by providing a publicly available, expertly curated knowledgebase of disease-specific variants and reference-cited evidence to the global clinical and research communities.
Trusted by Industry Leaders Including














Additional Resources
Estimation of ENPP1 deficiency genetic prevalence using a comprehensive literature review and population databases - Orphanet Journal of Rare Diseases
This paper reveals how Genomenon determined the genetic prevalence of ENPP1 deficiency to be 3X the previous estimate.
Novel PHEX Gene Locus‐Specific Database: Comprehensive Characterization of Vast Number of Variants Associated with X‐linked Hypophosphatemia (XLH)- Human Mutation
In this paper, with Ultragenyx, Genomenon provided a comprehensive literature review.
Uncovering Pathogenic GLA Variants in Fabry Disease: A Systematic Literature Review Using Real-World Evidence. - ACMG 2025
Genomenon provided a comprehensive literature review using Mastermind, a database of variants with evidence cited in the medical literature.
ENPP1 Deficiency: A Clinical Update on the Relevance of Individual Variants Using a Locus-Specific Patient Database. - Human Mutation
Reveals how creating a comprehensive database of curated variants that is widely accessible is crucial for increasing the diagnosis of ENPP1 Deficiency.